Each day we prescribe antibiotics without knowing the specific cause of infection, yet. Some patients will have an infection caused by an ESBL-producing bug, and they would benefit from immediate treatment with a carbapenem or addition of an aminoglycoside. At the same time we don’t want to misuse carbapenems or hurt kidneys. Wouldn’t it be great if we could accurately predict who would need a carbapenem? Now you can. Continue reading
epidemiology
Can we really halve Gram-negative BSIs (GNBSIs) by 2021? Kiernan vs. Otter Mk II
The UK government has recently announced their ambition to halve the rate of Gram-negative BSIs by 2021. Looking at the latest mandatory reporting dataset (see Figure 1 below), you can see why. Impressive reductions in MRSA BSI and C. difficile, but a notable increase in E. coli BSI. And this combined this with worrying data around increased antimicrobial resistance in Gram-negative bacteria from the ESPAUR report. In this post, Martin Kiernan and Jon Otter present both sides of the argument as to whether Gram-negative BSIs can be reduced by 2021, with comment from Andreas Voss and Marc Bonten! And you get to vote on which side of the argument you come down on after reading the arguments. Let battle commence…
How much S. aureus is hospital acquired? Mk II
I posted a blog a couple of years ago (was it really that long!) on a fascinating study suggesting that only 1/5 of S. aureus in hospital patients is hospital-acquired. My key conclusion from that study was that the number of potential sources for S. aureus that the team investigated was inadequate to draw any firm conclusions (they didn’t include staff, surfaces, or visitors). I concluded that ‘the next frontier of transmission mapping must be a more comprehensive evaluation of other potential sources…’. The authors must have been reading, because this study from the same group was published recently in Lancet ID, which is a more comprehensive evaluation of other potential sources.
Time to go shopping for a UVC system?
It is great to see the long-awaited ‘Benefits of Terminal Room Disinfection’ (BETR-D) randomised controlled trial of a UVC automated room decon (ARD) system published, in the Lancet, no less! This study firms up the importance of environmental contamination in transmission, and demonstrates additional benefit of UVC over and above enhanced conventional methods for VRE, maybe for MRSA, but not for C. difficile.
The silent Mycobacterium chimaera epidemic
There has been much discussion about the risk of Mycobacterium chimaera infections associated with contaminated heater-cooler units (HCUs) used in cardiothoracic surgery. A study published recently in CID explores the risk in the UK, and provides further evidence to link these tricky-to-treat infections to contaminated HCUs.
Chased by an antibiotic-induced C difficile-shaped shadow
A fascinating new JAMA Internal Medicine study suggests that being admitted to a room when the prior occupant had taken antibiotics increases the risk of the subsequent occupant of the same room developing C. difficile infection (CDI). Quite a few convincing epi studies have showed that admission to a room when the prior occupant was known to have a number of key pathogens (including C. difficile) increased the chance of acquisition for the subsequent occupant. But this study extends the ‘prior room occupancy’ concept into a new dimension!
Community MRSA preys on the poor and deprived
As you can probably tell from the title, this post comes with a warning: it presents some rather “un-PC” data, but I’ll do my best to deliver it calmly and dispassionately! My old research team from KCL have just published a paper in PLOS Medicine on the association between social and material deprivation, and MRSA.
I’ve been interested in the dynamic between hospital-associated (HA) and community-associated (CA) MRSA for years (not least because it was the subject of my PhD thesis). I wrote a review several years ago on how community MRSA should be seen as a genotypic phenomenon with epidemiological implications. Using this framework, it is possible to get your head around CA strains of MRSA beginning to cause hospital-acquired infections. The aim of this study was to use a large collection of MRSA from across several regions of London to explore the transmission dynamics and epidemiological associations of HA and CA types of MRSA.
Hot stuff?
 So I’m really quite interested in seasonality of infections. I first became interested in it when looking at increases in E. coli bacteraemia for ARHAI (report here) because of Jennie Wilson’s excellent paper showing seasonality of gram negative bacteraemia, echoed by similar observations and conjecture on warmer weather, more infection. This is true in hospitals as well as the community. Why would this be? We have tussled with increasing E. coli bacteraemia in the UK for a few years now. Goes up every summer, does not return to the baseline, goes up again next summer etc., etc.. Other countries have also reported this. I have heard some suggest this is due to longer hours of daylight leading to more barbeques and more sexual activity. Given that the majority of infections in the UK are >70 years of age, my senior years have no fears for me then.
So I’m really quite interested in seasonality of infections. I first became interested in it when looking at increases in E. coli bacteraemia for ARHAI (report here) because of Jennie Wilson’s excellent paper showing seasonality of gram negative bacteraemia, echoed by similar observations and conjecture on warmer weather, more infection. This is true in hospitals as well as the community. Why would this be? We have tussled with increasing E. coli bacteraemia in the UK for a few years now. Goes up every summer, does not return to the baseline, goes up again next summer etc., etc.. Other countries have also reported this. I have heard some suggest this is due to longer hours of daylight leading to more barbeques and more sexual activity. Given that the majority of infections in the UK are >70 years of age, my senior years have no fears for me then.
Do you know your CRE from your CRAB?
I gave a talk today at a meeting on combating carbapenem-resistant organisms. My angle was to clearly differentiate the epidemiology of the Enterobacteriaceae (i.e. CRE) from the non-fermenters (most importantly carbapenem-resistant A. baumannii – CRAB), and you can download my slides here.
I’ve blogged before about how confusing the terminology surrounding multidrug-resistant Gram-negative rods has become. Non-expert healthcare workers have little chance in distinguishing CRE from CPE from CRO from CPO. So we need to help them by developing some clear terminology, given the gulf in epidemiology between CRE and CRAB (see below).
CRE and CRAB are like apples and pears: they share some basic microbiology but that’s about where the comparison ends!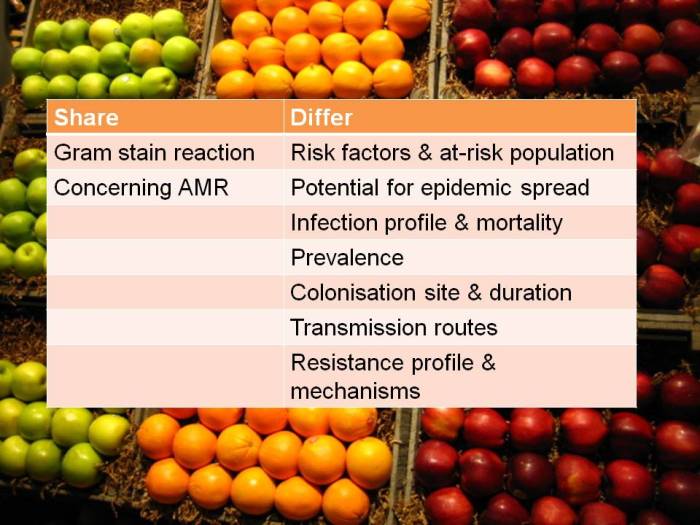
So, I think we should talk in terms of CRE (and CPE for confirmed carbapenemase carriers), and CRNF (or CRAB for A. baumannii and CRPA for P. aeruginosa). I don’t think that CRO is a useful term – in fact, I find it rather confusing. Carbapenem resistance in Enterobacteriaceae (CRE) and A. baumannii (CRAB) are both emerging problems, but they are not the same problem.
How big is C. difficile infection in the USA?
 The New England Journal of Medicine recently published an article evaluating the burden of CDI in the USA. The huge CDC-led initiative collected data from 10 geographically distinct regions, identifying more than 15,000 cases. Around two-thirds of cases were classified as healthcare-associated (although only 25% were hospital-onset). This means that, prima facie, a third of CDI cases were community-associated. I find this proportion difficult to believe: I strongly suspect that many of these cases would have had healthcare-associated risk factors if the team were able to look hard enough. For example, they used a fairly standard 12 week look-back period to evaluate previous hospitalisation, but how would the data look if they’d used 12 months? Also, it’s usually only possible to evaluate previous hospitalisation in a single healthcare system, but many patients commute between various healthcare systems. The authors acknowledge in the discussion that this designation of “community-acquired” may be inaccurate based on the finding from a previous study whether healthcare-associated risk factors were identified in most patients, but only be a detailed phone interview.
The New England Journal of Medicine recently published an article evaluating the burden of CDI in the USA. The huge CDC-led initiative collected data from 10 geographically distinct regions, identifying more than 15,000 cases. Around two-thirds of cases were classified as healthcare-associated (although only 25% were hospital-onset). This means that, prima facie, a third of CDI cases were community-associated. I find this proportion difficult to believe: I strongly suspect that many of these cases would have had healthcare-associated risk factors if the team were able to look hard enough. For example, they used a fairly standard 12 week look-back period to evaluate previous hospitalisation, but how would the data look if they’d used 12 months? Also, it’s usually only possible to evaluate previous hospitalisation in a single healthcare system, but many patients commute between various healthcare systems. The authors acknowledge in the discussion that this designation of “community-acquired” may be inaccurate based on the finding from a previous study whether healthcare-associated risk factors were identified in most patients, but only be a detailed phone interview.
Scaling up from the figures from the 10 regions, national estimates were around 500,000 cases and 29,000 deaths due to CDI per annum in the US. This estimate is approximately double previous estimates for the national CDI burden in the USA, probably reflecting the adoption of molecular methods for the detection of CDI. This scaling up included an interesting statistical adjustment to see how prevalence varied depending on how many sites use sensitive molecular methods to detect CDI.
A sub-study included the culture of C. difficile from 1625 patients. More than 15% of stool specimens from patients diagnosed as CDI failed to grow C. difficile, probably illustrating the limitations of culture methods more than anything else. NAP1 (027) represented around half of cases, and was significantly more common in healthcare-associated CDI. I think it’s fair to say that the initial fears that NAP1 was a super-strain have been allayed by the fact that it’s now so common and there hasn’t been a surge in CDI mortality.
Finally, around 21% of healthcare-associated cases suffered at least one recurrence. Thus, there is a real need to the roll out of the uber successful faecal microbiota transplantation for recurrent CDI. In fact, there should be around 70,000 faecal microbiota transplantations each year in the US right now (500,000 x 0.66 x 0.21); I suspect there are far fewer.


