The invisible menace! I’ve often thought it would be great if there was some visible sign that your hands had become contaminated during patient care. I guess that does happen to a degree when hands are visibly soiled – and we know that compliance with hand hygiene is almost universal when that happens. But what about when there’s no visible contamination but invisible and risky contamination with pathogens that can cause HCAI? A helpful systematic review and meta-analysis from 2019 suggests that around 5-10% of HCW working in acute care hospitals or care homes are contaminated with key hospital pathogens.
Continue readingAcinetobacter
How dirty is your QWERTY?
I was recently involved in a study to examine the microbial profile of computer keyboards in a multi-centre study in the UK. The findings have just been published in the Journal of Hospital Infection.
Continue readingWHO guidelines for the prevention and control of carbapenem-resistant organisms
WHO have just released some guidelines for the prevention and control of carbapenem-resistant Enterobacteriaceae, Acinetobacter baumannii, and Pseudomonas aerugionsa. This guideline builds on the excellent WHO core components for IPC guidelines.
UK guidelines for the control of multidrug-resistant Gram-negative bacteria
The UK guidelines for the prevention and control of multidrug-resistant Gram-negative bacteria (MDR-GNB) are published this week. It’s useful that the publication of these guidelines coincides with Antibiotic Awareness Week because MDR-GNB are brining us ever closer to the end of antibiotics. Although the guidelines don’t cover the treatment of MDR-GNB (this will be addressed in a forthcoming guideline), these highly resistant MDR-GNB leave few therapeutic options. Even when they remain susceptible to some antibiotics, these antibiotics are not front-line antibiotics for a reason (including poor tissue penetration and side effects). Furthermore, we are already seeing resistance to last-line (aka end of the golden-antibiotic-road) antibiotics e.g. colistin. Therefore, the old adage that ‘prevention is better than cure’ has never been so true!
Guidelines to control multidrug-resistant Gram-negative bacteria: an ‘evidence-free zone’
I recently had a review published in CMI comparing EU guidelines for controlling multidrug-resistant Gram-negative bacteria (MDR-GNB). I included the following guidelines in my review: ECCMID 2014, Irish MDRO, PHE CPE, HPS CPE, ECDC systematic review on CPE (not strictly a guideline, but did include some recommendations). A couple of important points arise:
European approaches to MDR-GNR prevention and control
I was privileged to be asked to speak at the inaugural Healthcare Infection Society Middle East Summit in Dubai this week on ‘European approaches to MDR-GNR prevention and control’. You can download my slides here.
I began with a (probably too lengthy) preamble outlining some overall points:
- CRE is a big deal in Europe, especially in the UK, and has prompted unprecedented action on a national level in the form of a Toolkit, a Patient Safety Alert and a letter to all CEOs requesting (demanding?) an action plan. The political picture is similar elsewhere in Europe and in the USA. Although this level of government scrutiny can be challenging, on the whole I think it’s beneficial, and is probably a sizeable factor in the successes achieved with MRSA and CDI.
- Do we go universal or targeted? There’s been much discussion recently about abandoning traditional targeted (aka vertical) approaches in favour of universal (aka horizontal). Interesting, all guidelines that I could lay my hands on favoured a targeted approach for MDR-GNR, centred around screening and isolation of carriers.
- Where is the evidence? We are hamstrung by the lack of high quality studies telling us with any certainty what works to control MDR-GNR. Pretty much all studies to date are either performed in an outbreak setting (regression to the mean…) or include multiple interventions (which worked?), or both. The few studies that evaluated a single intervention in an endemic setting are underpowered to deliver a meaningful conclusion. So, we need bigger and better studies!
- How do you produce good guidelines – who is on the guideline writing dream team, and what are the key pitfalls to avoid. Plus, importantly, how to good guidelines translate through a good policy into good practice?
Do you know your CRE from your CRAB?
I gave a talk today at a meeting on combating carbapenem-resistant organisms. My angle was to clearly differentiate the epidemiology of the Enterobacteriaceae (i.e. CRE) from the non-fermenters (most importantly carbapenem-resistant A. baumannii – CRAB), and you can download my slides here.
I’ve blogged before about how confusing the terminology surrounding multidrug-resistant Gram-negative rods has become. Non-expert healthcare workers have little chance in distinguishing CRE from CPE from CRO from CPO. So we need to help them by developing some clear terminology, given the gulf in epidemiology between CRE and CRAB (see below).
CRE and CRAB are like apples and pears: they share some basic microbiology but that’s about where the comparison ends!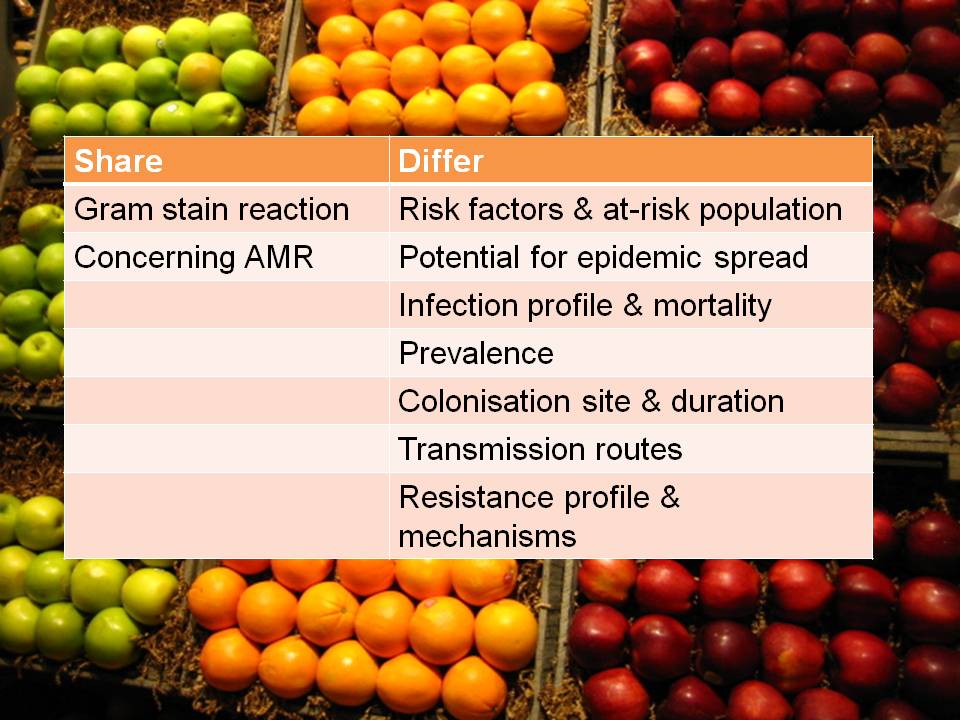
So, I think we should talk in terms of CRE (and CPE for confirmed carbapenemase carriers), and CRNF (or CRAB for A. baumannii and CRPA for P. aeruginosa). I don’t think that CRO is a useful term – in fact, I find it rather confusing. Carbapenem resistance in Enterobacteriaceae (CRE) and A. baumannii (CRAB) are both emerging problems, but they are not the same problem.
Filling the gaps in the guidelines to control resistant Gram-negative bacteria
I gave the third and final installment of a 3-part webinar series on multidrug-resistant Gram-negative rods for 3M recently. You can download my slides here, and access the recording here.
During the webinar, I provided an overview of the available guidelines to control CRE and other resistant Gram-negative bacteria. I then identified gaps in the guidelines, in terms of definitions of standard precautions, outbreak epidemiology and who should be on the guidelines writing dream team. Finally, I discussed some controversial areas in terms of effective interventions: patient isolation, staff cohorting and selective digestive decontamination.
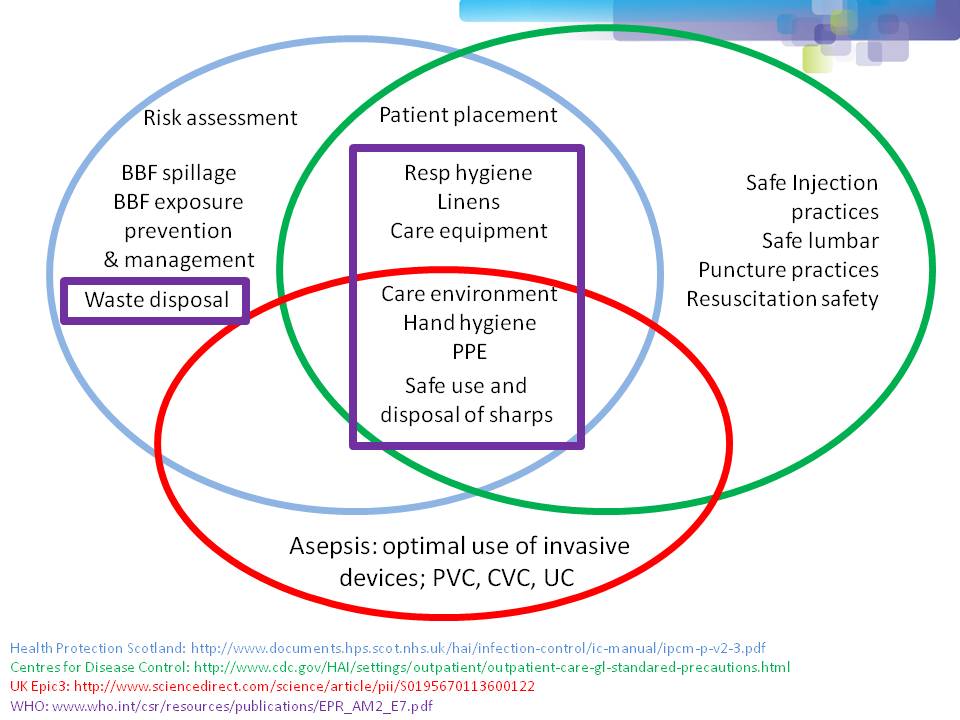
One of the most important points when considering infection prevention and control guidelines is the issue of ‘standard precautions’. What do we apply to every patient, every time? As you can see from Figure 1 below, ‘standard precautions’ is far from standardized. This is problematic when developing and implementing prevention and control guidelines.
Figure 1: differences in the definition of ‘standard precautions’.
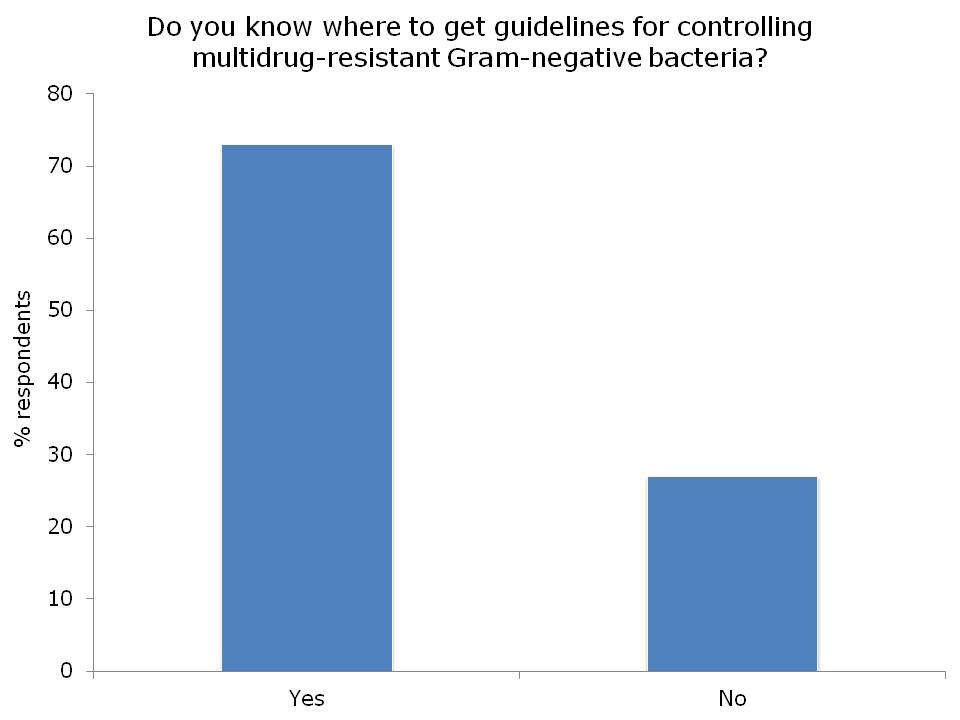
I had the opportunity to ask the webinar audience a few questions throughout the webinar, which are outlined in Figure 2.
Figure 2: response to the questions from the 120 or so participants.
I was somewhat concerned but not that surprised that more than a quarter of the audience did not know where to access control guidelines for MDR-GNR. I suppose this means that we need to do a better job of signposting the location of the various guidelines available. Here’s a non-exhaustive list for starters:
- US CDC CRE Toolkit.
- US AHRQ CRE Toolkit.
- UK Public Health England CPE Tookit.
- UK ESBL guidelines.
- ECDC risk assessment on the spread of spreading (CPE).
- Canadian guidelines for carbapenem resistant GNB.
- Australian recommendations for CRE control.
- ESCMID MDR-GNR control guidelines.
There was a fairly even split between active and passive surveillance to detect outbreaks. The problem with relying on passive surveillance (i.e. clinical cultures) is that there’s a good chance that the ‘horse will have bolted’, and you have a large outbreak on your hands, before a problem is detected. For this reason, I favour active surveillance.
But who to screen? In the case of CRE, I was pleased to see that virtually nobody said nobody. There was a pretty even split between everybody, high-risk individuals or all individuals in high-risk specialties. Accurately identifying individuals who meet screening triggers is operationally challenging, as outlined by the “backlash” to the UK toolkit, so I think screening all patients in high-risk specialties (e.g. ICU) makes most sense.
So, what works to control MDR-GNR transmission? We don’t really know, so are left with a “kitchen sink” (aka bundle approach) (more on this in my recent talk at HIS). We need higher quality studies providing some evidence as to what actually works to control MDR-GNR. Until then, we need to apply a healthy dose of pragmatism!
Being bitten by antibiotic resistant CRAB hurts! (Acinetobacter that is.)
Guest bloggers Dr. Rossana Rosa and Dr. Silvia Munoz-Price (bios below) write…
In everyday practice of those of us who work in intensive care units, a scenario frequently arises: a patient has a surveillance culture growing carbapenem-resistant Acinetobacter baumannii (CRAB). While the ultimate course of action we take will be dictated by the patient’s clinical status, that surveillance culture, in the appropriate context, can provide us with valuable information.
For this study1, we looked at a cohort of patients admitted to a trauma intensive care unit, and sought to identify the risk factors for CRAB infections. We found that patients who had surveillance cultures positive for CRAB had a hazard ratio of 16.3 for the development of clinical infections with this organism, compared to patient’s who remained negative on surveillance, even after adjusting for co-morbidities and antibiotic exposures. Since our results were obtained as part of a well-structured surveillance program, we know that colonization preceded infection. Unfortunately for some of our patients, the time from detection of colonization to development of clinical infections was a matter of days. With therapeutic options for the effective treatment of infections with CRAB limited to tigecycline and polymixins, the consequences of delaying therapy are often fatal. As described by Lee et al, a delay of 48 hour in the administration of adequate therapy for CRAB bacteremia can result in a 50% difference in mortality rate2.
Surveillance cultures are not perfect, and may not detect all colonized patients, but they can be valuable tools in the implementation of infection control strategies3, and as we found in our study, can also potentially serve to guide clinical decision that impact patient care and even survival.
Bio:
Dr. Silvia Munoz-Price (centre left) is an Associate Professor of Clinical Medicine at the Institute for Health and Society, Medical College of Wisconsin, currently serving as the Enterprise Epidemiologist for Froedert & the Medical College of Wisconsin. Dr. Rossana Rosa (centre right) is currently an Infectious Diseases fellow at Jackson Memorial Hospital-University of Miami Miller School of Medicine. She hopes to continue developing her career in Hospital Epidemiology and Infection Control.
References
- Latibeaudiere R, Rosa R, Laowansiri P, Arheart K, Namias N, Munoz-Price LS. Surveillance cultures growing Carbapenem-Resistant Acinetobacter baumannii Predict the Development of Clinical Infections: a Cohort Study. Clin Infect Dis. Oct 28 2014.
- Lee HY, Chen CL, Wu SR, Huang CW, Chiu CH. Risk factors and outcome analysis of Acinetobacter baumannii complex bacteremia in critical patients. Crit Care Med. May 2014;42(5):1081-1088.
- Munoz-Price LS, Quinn JP. Deconstructing the infection control bundles for the containment of carbapenem-resistant Enterobacteriaceae. Curr Opin Infect Dis. Aug 2013;26(4):378-387.
Image: Acinetobacter.
Reflections from HIS 2014, Part II: Dealing with the contaminated environment
Welcome to Part II of my reflections from HIS. For the box-set, see the list at the beginning of Part I here.
Dr Karen Vickery – Multispecies biofilms on dry hospital surfaces – harbouring and protecting multiantibiotic resistant organisms
Probably the most important update from the entire conference was more data from the Vickery lab on biofilms on dry hospital surfaces. She excised 44 dry surface samples from the ICU, put them under the electron microscope and, lo and behold, 41 of them (93%) had fully-fledged (if somewhat unusual) EPS-producing biofilms on! The implications are huge: this could explain extended surface survival, poor success rate of surface sampling, and result in reduced biocide susceptibility up to the tune of 1000x (see my review just published in JHI with Karen as a co-author for more on biocides and biofilm susceptibility).
Dr Silvia Munoz-Price – Controlling multidrug resistant Gram-negative bacilli in your hospital: We can do it so can you!
Dr Munoz-Price described her hospital’s impressive reductions on carbapenem-resistant A. baumannii – from 12 new isolates per week to virtually none today. So what worked? It’s difficult to be sure since it was a bundled intervention. Dr Munoz-Price described the rationale behind some elements of the bundle: environmental surface and staff hand sampling to visualize the invisible, environmental cleaning and disinfection to deal with the ‘fecal [sic] patina’ [a stooly veneer emanating from the rectum] (see Dr Munoz-Price and Dr Rosa’s guest blog for more details), and chlorhexidine bathing. Perhaps the most interesting aspect was the various implementation challenges that were overcome. It was amazing how far removed practice ‘in the trenches’ was from the policy set by the epidemiologist’s office, exemplified by environmental staff buying their own UV lamps to for “spot cleaning” removal of fluorescent markers of cleaning thoroughness. Overcoming these challenges required more that the stick (citations for non-compliance, which failed); culture change takes understanding, time and a very large carrot (and some sticks too, sometimes).
Jim Gauthier – faeces management
A number of key pathogens are associated with faecal colonization and shedding: C. difficile, VRE, ESBL and CRE. Jim didn’t mention MRSA, but this can also cause gastrointestinal colonization and, more controversially, infection. Enterobacteriaceae can survive on dry surfaces for longer than you’d expect, too. We traditionally worry about surface contamination of high-touch sites in inpatient settings. Floor contamination isn’t important (unless you happen to be a wheel chair user, a toddler, or drop your pen). Contamination in outpatient settings isn’t a problem either (unless you happen to have a fairly short consultation for a patient with VRE). So, what to do? Jim introduced the idea of a ‘hierarchy of control’; put another way, prevention is better than cure, so do we have the right systems in place to manage faeces which is teeming with hospital pathogens? For example, should we be enforcing mandatory contact precautions for all contact with faeces (standard precautions – which aren’t very standard anyway – are probably not adequate)? Finally, Jim mentioned the growing importance of faecal microbiota transplantation (and hearing a Canadian speak about this reminded me of a hilarious spoof video).
No-touch automated room decontamination (NTD)
 Figure: Hospital bed rails are frequently contaminated, and often not easy to clean and disinfect using conventional methods.
Figure: Hospital bed rails are frequently contaminated, and often not easy to clean and disinfect using conventional methods.
Paul Dickens – establishing Ebola surge isolation capacity in the UK
Paul Dickens gave a whistle-stop overview of the detailed plans for Ebola surge capacity in the UK (perish the thought). He began by describing the replacement of formaldehyde with hydrogen peroxide vapour for the decontamination of the patient isolators at the Royal Free High Level Isolation Unit (HLIU). They now have a tried and tested process and protocols in place to get the HLIU back online within days using hydrogen peroxide vapour decontamination, where the previous protocol using formaldehyde put it out of action for 6 weeks! (I was involved in writing the protocols for this tricky decontamination assignment, which were reported on a poster published at HIS.) Other challenges in establishing surge capacity include staff expertise, and PPE recommendations, supply & training. Surge capacity is now established. Let’s just hope we won’t need it!
Dr Frédéric Barbut – How to eradicate Clostridium difficile spores from the environment
There’s now plenty of evidence that contaminated surfaces contribute to the transmission of C. difficile. These environmental intervention studies show a 50-80% reduction in the rate of CDI; does this mean that 50-80% of CDI acquisition is environmentally-associated? This seems too high, but it’s difficult to think of another explanation. Furthermore, there is emerging but compelling evidence of a proportional relationship between the degree of C. difficile surface contamination and transmission risk? I really don’t think that the public have yet ‘got’ that the previous occupant can influence acquisition risk. And when they do, I think there will be increasing demand for properly decontamination rooms. So, is it time to turn to NTD systems? Sometimes, yes. And do you go for hydrogen peroxide or UV? Well, that depends on what you’re trying to achieve! If you’re trying to eliminate pathogens, which sometimes you will be, then hydrogen peroxide vapour is the best choice. But if you’re trying to reduce contamination levels without necessarily eliminating all pathogens, then UV is the best choice due to its speed and ease of use.
The debate: “Hospitals that do not use high-tech decontamination of the environment are doing their patients a disservice.”
This debate pitted Profs Hilary Humphreys and Phil Carling (pro) against Peter Hoffman and Martin Kiernan (con). It was lively, entertaining and engaging…
Prof Humphreys argued that it is not acceptable to admit patients to rooms with inherent additional risk for transmission. We can address this by ‘walking like the Egyptians’ and copperising our surfaces, for which there is now some data with a clinical outcome. Another approach is NTD systems, for which data (including some clinical outcomes) are emerging. Prof Carling’s presentation was somewhat unusual, with his arguments seemingly an appeal to common sense rather than drawn from the published literature.
Martin Kiernan began by acknowledging the role of the environment, but that hand contamination is almost always the final vector (and there’s some evidence for this). The cornerstone of Martin’s argument was that whether NTD systems work is the wrong question. We should be focusing our time, money and attention on improving conventional methods which have been shown to reduce transmission. Peter Hoffman complemented Martin’s pragmatic viewpoint with thorough, thoughtful critiques of the studies on HPV decontamination with a clinical outcome. The 2008 Boyce study has more holes than the 2013 Passaretti study, which itself is far from watertight!
The key argument for turning to NTD systems is that admission to a room previously occupied by a patient with an MDRO increases the risk of acquisition due to residual contamination, and NTD decontamination mitigates this increased risk. So, my own conclusion is that hospitals that do not use high-tech decontamination of the environment are indeed doing their patients a disservice. Sometimes!
Look out for the third and final installment of my reflections from HIS 2014 at some point tomorrow!