Welcome to Part II of my reflections from HIS. For the box-set, see the list at the beginning of Part I here.
Dr Karen Vickery – Multispecies biofilms on dry hospital surfaces – harbouring and protecting multiantibiotic resistant organisms
Probably the most important update from the entire conference was more data from the Vickery lab on biofilms on dry hospital surfaces. She excised 44 dry surface samples from the ICU, put them under the electron microscope and, lo and behold, 41 of them (93%) had fully-fledged (if somewhat unusual) EPS-producing biofilms on! The implications are huge: this could explain extended surface survival, poor success rate of surface sampling, and result in reduced biocide susceptibility up to the tune of 1000x (see my review just published in JHI with Karen as a co-author for more on biocides and biofilm susceptibility).
Dr Silvia Munoz-Price – Controlling multidrug resistant Gram-negative bacilli in your hospital: We can do it so can you!
Dr Munoz-Price described her hospital’s impressive reductions on carbapenem-resistant A. baumannii – from 12 new isolates per week to virtually none today. So what worked? It’s difficult to be sure since it was a bundled intervention. Dr Munoz-Price described the rationale behind some elements of the bundle: environmental surface and staff hand sampling to visualize the invisible, environmental cleaning and disinfection to deal with the ‘fecal [sic] patina’ [a stooly veneer emanating from the rectum] (see Dr Munoz-Price and Dr Rosa’s guest blog for more details), and chlorhexidine bathing. Perhaps the most interesting aspect was the various implementation challenges that were overcome. It was amazing how far removed practice ‘in the trenches’ was from the policy set by the epidemiologist’s office, exemplified by environmental staff buying their own UV lamps to for “spot cleaning” removal of fluorescent markers of cleaning thoroughness. Overcoming these challenges required more that the stick (citations for non-compliance, which failed); culture change takes understanding, time and a very large carrot (and some sticks too, sometimes).
Jim Gauthier – faeces management
A number of key pathogens are associated with faecal colonization and shedding: C. difficile, VRE, ESBL and CRE. Jim didn’t mention MRSA, but this can also cause gastrointestinal colonization and, more controversially, infection. Enterobacteriaceae can survive on dry surfaces for longer than you’d expect, too. We traditionally worry about surface contamination of high-touch sites in inpatient settings. Floor contamination isn’t important (unless you happen to be a wheel chair user, a toddler, or drop your pen). Contamination in outpatient settings isn’t a problem either (unless you happen to have a fairly short consultation for a patient with VRE). So, what to do? Jim introduced the idea of a ‘hierarchy of control’; put another way, prevention is better than cure, so do we have the right systems in place to manage faeces which is teeming with hospital pathogens? For example, should we be enforcing mandatory contact precautions for all contact with faeces (standard precautions – which aren’t very standard anyway – are probably not adequate)? Finally, Jim mentioned the growing importance of faecal microbiota transplantation (and hearing a Canadian speak about this reminded me of a hilarious spoof video).
No-touch automated room decontamination (NTD)
 Figure: Hospital bed rails are frequently contaminated, and often not easy to clean and disinfect using conventional methods.
Figure: Hospital bed rails are frequently contaminated, and often not easy to clean and disinfect using conventional methods.
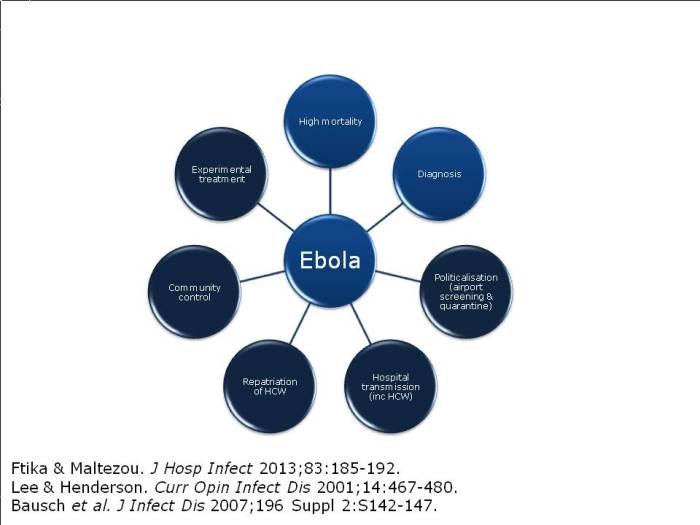
Paul Dickens – establishing Ebola surge isolation capacity in the UK
Paul Dickens gave a whistle-stop overview of the detailed plans for Ebola surge capacity in the UK (perish the thought). He began by describing the replacement of formaldehyde with hydrogen peroxide vapour for the decontamination of the patient isolators at the Royal Free High Level Isolation Unit (HLIU). They now have a tried and tested process and protocols in place to get the HLIU back online within days using hydrogen peroxide vapour decontamination, where the previous protocol using formaldehyde put it out of action for 6 weeks! (I was involved in writing the protocols for this tricky decontamination assignment, which were reported on a poster published at HIS.) Other challenges in establishing surge capacity include staff expertise, and PPE recommendations, supply & training. Surge capacity is now established. Let’s just hope we won’t need it!
Dr Frédéric Barbut – How to eradicate Clostridium difficile spores from the environment
There’s now plenty of evidence that contaminated surfaces contribute to the transmission of C. difficile. These environmental intervention studies show a 50-80% reduction in the rate of CDI; does this mean that 50-80% of CDI acquisition is environmentally-associated? This seems too high, but it’s difficult to think of another explanation. Furthermore, there is emerging but compelling evidence of a proportional relationship between the degree of C. difficile surface contamination and transmission risk? I really don’t think that the public have yet ‘got’ that the previous occupant can influence acquisition risk. And when they do, I think there will be increasing demand for properly decontamination rooms. So, is it time to turn to NTD systems? Sometimes, yes. And do you go for hydrogen peroxide or UV? Well, that depends on what you’re trying to achieve! If you’re trying to eliminate pathogens, which sometimes you will be, then hydrogen peroxide vapour is the best choice. But if you’re trying to reduce contamination levels without necessarily eliminating all pathogens, then UV is the best choice due to its speed and ease of use.
The debate: “Hospitals that do not use high-tech decontamination of the environment are doing their patients a disservice.”
This debate pitted Profs Hilary Humphreys and Phil Carling (pro) against Peter Hoffman and Martin Kiernan (con). It was lively, entertaining and engaging…
Prof Humphreys argued that it is not acceptable to admit patients to rooms with inherent additional risk for transmission. We can address this by ‘walking like the Egyptians’ and copperising our surfaces, for which there is now some data with a clinical outcome. Another approach is NTD systems, for which data (including some clinical outcomes) are emerging. Prof Carling’s presentation was somewhat unusual, with his arguments seemingly an appeal to common sense rather than drawn from the published literature.
Martin Kiernan began by acknowledging the role of the environment, but that hand contamination is almost always the final vector (and there’s some evidence for this). The cornerstone of Martin’s argument was that whether NTD systems work is the wrong question. We should be focusing our time, money and attention on improving conventional methods which have been shown to reduce transmission. Peter Hoffman complemented Martin’s pragmatic viewpoint with thorough, thoughtful critiques of the studies on HPV decontamination with a clinical outcome. The 2008 Boyce study has more holes than the 2013 Passaretti study, which itself is far from watertight!
The key argument for turning to NTD systems is that admission to a room previously occupied by a patient with an MDRO increases the risk of acquisition due to residual contamination, and NTD decontamination mitigates this increased risk. So, my own conclusion is that hospitals that do not use high-tech decontamination of the environment are indeed doing their patients a disservice. Sometimes!
Look out for the third and final installment of my reflections from HIS 2014 at some point tomorrow!