We recently published a study in the Journal of Antimicrobial Chemotherapy relating the impact of introducing an enhanced testing* programme for CPE in London. (And yes, this is the first post for a while that isn’t on COVID-19!) Following an outbreak of NDM-producing Klebsiella pneumoniae affecting 40 patients in 2015 (published elsewhere, here and here), we ramped up our CPE testing programme. The number of patients carrying CPE increased substantially, from around 10 patients per month in June 2015 to around 50 per month in March 2018. However, the proportion of tests that were positive for CPE remained constant at around 0.4%, suggesting this was more effective carrier identification rather than a swelling pool of carriers per se; seek and ye shall find! Curiously, the majority of CPE identified were not linked in time and space with other CPE, suggested they represented a ground-swell of CPE coming into the hospital, rather than frequent in-hospital transmission. Also, the number of patients with CPE infections during the study period did not increase, which was reassuring.
prevalence
No CPE but a lot of VRE
Addenbrookes hospital in Cambridge (UK) have recently performed a point prevalence survey for antibiotic resistant bacteria. None of 540 patient samples grew CPE, but 130 (24%) grew VRE. So, why no CPE but so much VRE?
Who’s looking for CPE in English hospitals?
A team of authors surveyed NHS acute hospitals in England to determine the approach to CPE detection, including laboratory methods. The findings provide an opportunity to compare the approach to CPE detection and prevalence nationally, identifying higher CPE prevalence in the North-West, North-East and the South-East (the region that includes London) of England. The findings also suggest that more screening for CPE would detect more carriers – and perhaps help to prevent a silent epidemic of CPE in some regions.
CPE: a good reason to avoid surgery in Spain!
A clear simple study has a stark headline: 16% of admissions to a Spanish surgical ICU carry CPE. This sort of carriage prevalence is at a ‘practice-affecting’ level: the empiric antibiotic choices may be altered and you begin to wonder what is left when the first signs of infection develop in almost 1 in every 5 patients…
ESPAUR 2016: an early Christmas present
I am just getting around to reading (well detail-scanning the exec summary) of the ESPAUR report. My main reflection is what a fantastic resource this reporting stream offers us: to have freely accessible, regular, accurate, national data on antimicrobial resistance and usage, and other related indicators is pretty unique!
Healthcare-Associated Infections (HAIs); at what cost?
“Healthcare-Associated Infections” (HAIs)* increase morbidity, mortality and length of hospital stay as well as healthcare costs for the patients, their families and healthcare systems.1 They also lead to long-term disability and increased resistance of microorganisms to antimicrobials. Various studies have attempted to estimate the burden of HAIs. In this context, I came across two recent papers2,3 estimating the cost of HAIs in US acute care hospitals, which prompted me to re-visit the excellent WHO report on the burden on HAIs around the world.1
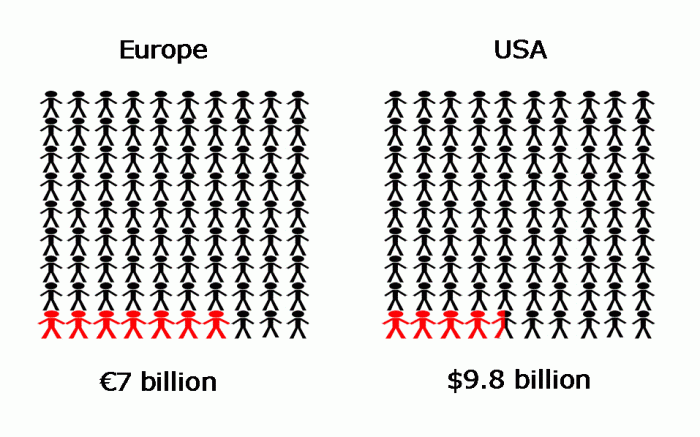
The WHO report explores the burden of HAIs not only in the high-income countries, where most reported burden estimates come from, but also in low- and middle-income countries, where little data are available. The report indicates that of every 100 hospitalised patients at any given time, 7 in developed and 10 in developing countries will acquired at least one HAI. The prevalence of HAIs in Europe is around 7.1% with more than 4 million patients affected by approximately 4.5 million episodes of HAIs annually and leading to 16 million extra-days of hospital stay, 37,000 attributable deaths and contributing to an additional 110,000. In the US around 1.7 million patients are affected by HAIs annually with a prevalence of 4.5% and accounting for 99,000 deaths. Limited data are available from low and middle-income countries but the prevalence of HAIs in these countries is estimated to be between 5.7 and 19.1%. Increased length of hospital stay associated with HAIs in developing countries range between 5-29.5 days and excess mortality due to these infections in adult patients in Latin America, Asia, Africa were 18.5%, 23.6%, and 29.3%, for CAUTI, CR-BSI, and VAP, respectively.1
HAIs have a huge economic burden. In the WHO report and according to a report from the ECDC,4 these infections account for approximately €7 billion per year in Europe, considering direct costs only. For instance, additional associated costs of a CR-BSI episode in Europe ranged from €4,200 to €13,030, representing annual costs to healthcare systems of €54 million in the United Kingdom and €130 million in France.1 Limited data on the financial cost of HAIs in low- and middle-income countries is available. Reports from Mexican ICUs estimated the overall average cost of a HAIs episode at $12,155 with an excess cost of $11,591 per case of CR-BSI.1 In several ICUs in Argentina, the overall extra cost estimates for CR-BSI and healthcare-associated pneumonia averaged $4,888 and $2,255 per case, respectively.1
In the US, the annual economic impact of HAIs was approximately $6.5 billion in 2004.5 Recently Zimlichman and colleagues2 conducted a systematic review of the literature for the years 1986 through 2013 for an updated estimate of costs associated with the most significant and targetable HAIs in the US. These were CLABSI, VAP, SSI, CR-UTI, and C. difficile infection (CDI). On a pair case basis, CLABSI were found to be the most costly at $45,814 (95% CI, $30,919-$65,245), followed by VAP at $40,144 (95% CI,$36,286-$44,220), SSI at $20,785 (95% CI, $18,902-$22,667), CDI at $11,285 (95% CI, $9,118-$13,574), and CR-UTI at $896 (95% CI, $603-$1,189). Based on 2009 data where approximately 34.7 million adults received inpatient care in US hospitals (totaling 165 million patient days), the total annual cost of the 5 infections was $9.8 billion (95% CI, $ 8.3-11.5 billion) with SSI and CDI being the most frequent (36% and 30% respectively).
Figure: The prevalence and direct cost of HAI in Europe1,4 and the USA.1,2
The study by Marchetti and Rossiter3 went a step further in trying to estimate the true cost of HAIs in US acute care hospitals by assessing the full social burden of these infections including direct medical, non-medical and indirect costs. This was done by updating, combining and expanding previous cost estimates from various studies. Although the study was subject to the same limitations as the studies which contributing data was derived from, it is of importance because the social cost of HAIs is rarely considered. Marchetti and Rossiter estimated the total social cost of HAIs in US acute care hospitals alone (excluding those occurring in non-hospital settings) to range from $96-147 billion. In the face of such a huge cost, the authors concluded “The enormous clinical and economic burden of infection places HAIs high on the list of devastating and costly illnesses, such as cancer, heart attack, stroke, and diabetes, thereby mandating further research and greater efforts to contain a pressing healthcare problem”.
It is clear that HAIs represent a huge burden in the developed world. Due to the limited data available from low- and middle-income countries, the true cost of these infections is undetermined, although it is clear that prevalence is higher in these countries. More research is needed to evaluate the true burden of HAIs worldwide, including their financial cost, to expose a problem that is as devastating and costly as cancer and diabetes. Needless to say, the implementation of practical and effective strategies to reduce the prevalence of HAIs is required.
*Healthcare-Acquired Infections, also known as “Healthcare-Associated Infections”, “Nosocomial Infections” or “Hospital Infections”, are infection acquired by patients in healthcare facilities or appear after discharge from a healthcare facility and are not present or incubating at the time of admission. Their definition also extends to occupational infections among healthcare workers.
Article citations:
- WHO. Report on the burden of endemic Health Care-Associated Infection Worldwide. WHO. 2011.
- Zimlichman E, Henderson D, Tamir O et al. Health Care-Associated Infections: A Meta-analysis of Costs and Financial Impact on the US Health Care System. JAMA Intern Med 2013.
- Marchetti A, Rossiter R. Economic Burden of Healthcare-Associated Infection in US Acute Care Hospitals – Societal Perspective. J Med Econ 2013.
- Annual epidemiological report on communicable diseases in Europe 2008. Report on the state of communicable diseases in the EU and EEA/EETA countries. Stockholm, ECDC. 2008.
- Klevens RM, Edwards JR, Richards CL Jr et al. Estimating health care-associated infections and death in US hospitals, 2002. Public Health Reports 2007;27:817-824.