I was asked to do a talk today on the modes of CPE transmission at a PHE Workshop on tackling CPE. It caused me to do a lot of thinking and write a new presentation, so I thought I’d share. You can download the slides here.
I divided my thoughts into two sections: CPE transmission modes (the physical ways in which CPE spreads) and CPE transmission modulators (the enablers and moderators of CPE spread).
CPE transmission modes
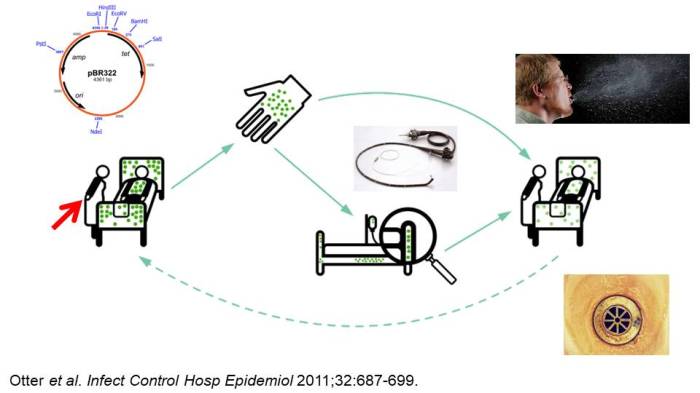
Figure 1: A summary of CPE transmission modes: hands, surfaces, medical devices for sure. But what about sinks / drains / wastewater, air, colonised staff, and plasmid spread?
Everybody would accept that contaminated hands, environmental surfaces, and medical devices can contribute to the spread of CPE at least some of the time. But there are other potential modes of CPE transmission that we need to consider:
- There’s an excellent section in the ECCMID MDR-GNB guidelines summarising the evidence that contaminated hands contributed to the transmission of Gram-negative bacteria. We know it’s a vital intervention, and yet compliance remains stubbornly around 40% (despite what many observational audits may say)!
- Contaminated surfaces. Although there is an old perception that only Gram-positive bacteria and Gram-negative non-fermenters (like Acinetobacter baumannii) can survive well on surfaces, some Enterobacteriaeae species also possess remarkable survival properties on dry surfaces. I did a study years ago where one strain of pneumoniae survive for >6 weeks with a minimal log reduction on a dry surface, in line with the ‘performance’ of C. difficile spores! So, all of the powerful epidemiological evidence about prior room occupancy and the risk of acquisition comes into play for CPE (although I don’t think anybody has yet published a study about CPE and prior room occupancy).
- Inadequately decontaminated medical devices. The now infamous outbreak of CPE associated with duodenoscopes in Illinois has changed the way that these scopes are managed. 39 patients were involved in the outbreak and exposure to these scopes resulted in a towering odds ratio of 78. This statement from the FDA in 2015 is telling: ‘Meticulously cleaning duodenoscopes prior to high-level disinfection should reduce the risk of transmitting infection, but may not entirely eliminate it. (FDA Feb 23 2015).’ This is an accepted risk that we need to manage.
- Sinks / drains / wastewater. I posted a few weeks ago about an American study highlighting surprising levels of carbapenemase contamination in hospital wastewater, and genetically linking environmental and patient isolates from hospital sinks. However, cause and effect is difficult to disentangle here. A new study helps here, finding that an intervention to improve the management of sinks in an ICU slashed the rate of CPE acquisition (by half!) and CPE clinical cultures (by two-thirds!!). Could it really be that two thirds of CPE clinical isolates are caused by contaminated sinks??
- Contaminated air. Whilst contaminated air isn’t going to be the main transmission mode for CPE, could it be a risk? We have learnt in recent years that difficile spores are commonly floating around in hospital air. I couldn’t find much evidence either way for CPE, but I did turn up this ID Week abstract finding that 4% of air cultures were positive for CPE in rooms of patients known to be CPE infected or colonised. More work needed here.
- Colonised staff. This is a Pandora’s box. Do we really want to open it? If there was a protracted outbreak and all else had failed, then we may have to. But given the limited decolonisation options for CPE, the outlook could be bleak for any colonised healthcare workers. A brave US study opened this particular Pandora’s box, and found a very low rate of CPE colonisation of staff: just 0.3% of 376 non-patient-facing staff, and none of 379 patient-facing staff. So, there may not be that much in this Pandora’s box (but still not wise to open it unless we really need to).
- Plasmid transmission. Inter-species plasmid transfer of AMR genes was investigated in the 1970s regarding gentamicin-resistance. There are several convincing individualsmall outbreaks where it is clear that inter-species plasmid transfer of AMR genes has occurred in Gram-negative bacteria. In one plasmid outbreak, multiple carbapenemases were dealt out like a pack of cards to multiple Enterobacteriaceae species from a single index patient. More broadly, the population structure of KPC-producing pneumoniae is consistent with horizontal gene transfer. So, we need to look beyond ‘same-bug-same-gene’ transmission dynamics.
CPE transmission modulators
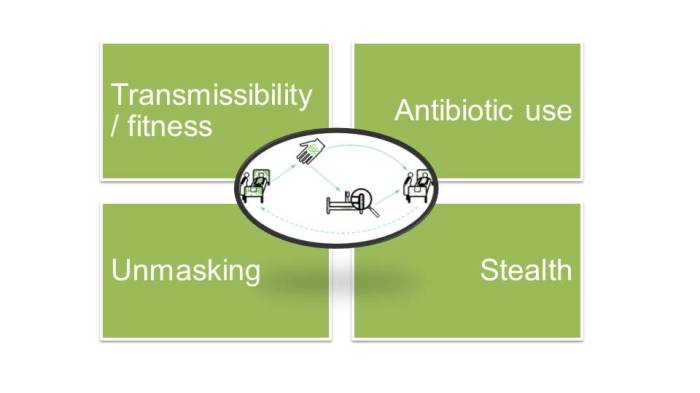
Figure 2: Some CPE transmission modulators.
So what factors enable or modulate the transmission of CPE through the modes outlined above (and I hope it goes without saying that basic IPC practice is one of them!). There are many. Some of the key ones include:
- Bacterial transmissibility / fitness. A recent study reported that Klebsiella species transmitted almost 4x more frequently than coli in a Dutch ICU setting. Why is this? Presumably because Klebsiella species are more fit to spread in the hospital setting. Is this also the case for different strains of Klebsiella species (almost certainly), and perhaps also for different carbapenemases in the same strain? Much more to learn here.
- Antibiotic use. There is no doubt that antibiotic use is a key driver of CPE. Overuse of antibiotics creates a strong local selective pressure that provides the incentive for bacteria to acquire carbapenemases. So perhaps we’re not necessarily talking about transmission, but about transition of antibiotic-susceptible to antibiotic-resistant versions of bacteria in hospitals. Take away this selective pressure, and quite quickly the levels of antibiotic resistance go down.
- What I mean here is that if we don’t have a good idea of who is colonised, the first we’ll hear of a sizable CPE outbreak is when a good number of patients are already CPE carriers and a few clinical infections (the age-old tip of the iceberg) are identified. This was the exact scenario in our 2015 outbreak of NDM-producing K. pneumoniae (as illustrated by the missing front half of the epi curve here). It wouldn’t surprise me is CPE is effectively in stealth mode throughout much of the country – and indeed the world (although nobody really knows how much screening is currently being undertaken).
- When a ‘new’ CPE case is detected, we need to keep in the back of our minds that it may not necessarily be a new acquisition even if the patient has a history of negative screens. There is some evidence that a “+-+” pattern of colonisation from repeated screens is common, probably due to variation in the concentration of gut colonisation oscillating above and below the limit of laboratory detection (exacerbated by antibiotics and other exposures), variation in the quality of the sample taken, and actual decolonisation and re-acquisition.
This is a complex, tricky picture, some of which is difficult to explain to our non-expert colleagues (especially the bits about plasmid transmission, and unmasking colonisation). We need more research in key areas to understand the fundamental science of CPE transmission modes and modulators. Until we have a strong evidence base for understanding CPE transmission, we just have to throw the kitchen sink (let’s hope it’s not contaminated) of IPC interventions at the problem in the hope that something will be effective.
Discover more from Reflections on Infection Prevention and Control
Subscribe to get the latest posts sent to your email.
Thank you for sharing this.
Kind regards,
Karina Nolte
Deskundige infectiepreventie
Tel.: 06-20681209
Aanwezig: Ma|Di|Wo|Do|Vr
[Tensen en Nolte infectiepreventie]
Deze boodschap is slechts bedoeld om u van informatie te voorzien. Aan de inhoud ervan kunnen geen rechten worden ontleend. Deze boodschap kan informatie bevatten die niet voor u bestemd is, dan wel vertrouwelijk is. Als u niet degene bent die als geadresseerde is genoemd, dan gaarne deze boodschap vernietigen zonder deze te lezen, te gebruiken, te dupliceren of de inhoud op welke manier dan ook aan anderen over te brengen.
LikeLike
Thanks Jon.
Would have liked to hear your presentation.
Hope you’re well
regards
Ann
LikeLike
Thank you Jon, good points in here and well communicated as per usual.
Did you know that Horizontal Gene Transfer via plasmid exchange occurs on some touch-surface materials and not on others? http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3509412/
It would be good to see the current market offering of antimicrobial touch-surface materials being tested to more meaningful efficacy test standards than ISO 22916 (or JIS Z 2801) which only measure antimicrobial efficacy under warm & wet conditions, with results measured over 24 hours…. which is not relevant to items such as lift buttons, blood-pressure cuffs, aseptic procedure trays, etc.
Use of effective antimicrobial touch-surface materials should of course only be an adjunct to hand hygiene and cleaning, but can be a simple and safe additional measure to rapidly and consistenly reduce microbial bioburden on fomites that regularly have high levels of contamination.
Welcome your thoughts on materials and test protocols, please.
LikeLike